Lesson 3
H2O, pH, Buffers, Functional Groups
We have a lot to get through this week, but these foundational concepts will help you understand lessons moving forward.
H2O
As we learned last week, water is a polar molecule that is able to form strong hydrogen bonds. These hydrogen bonds allow water to have unique properties such as high heat capacity, high heat of vaporization, and being an excellent solvent. Because our body is largely water (60-70%!) it is important to understand these characteristics. Read this page from Lumen Learning about the properties of water (stop when you get to the pH section).
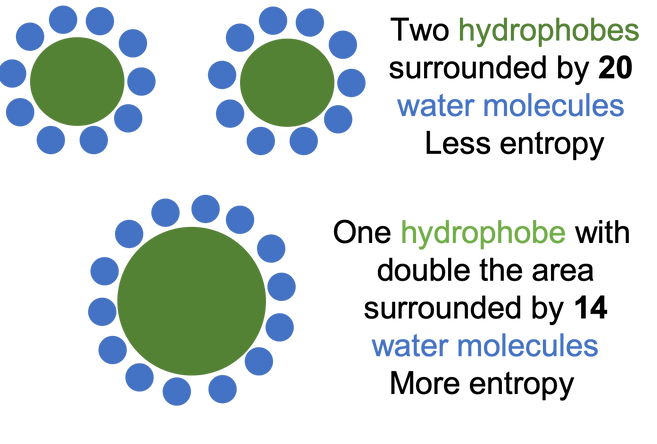
Another important consequence of the properties of water is that they give rise to the hydrophobic effect. We could probably spend an entire lesson on the hydrophobic effect itself, but we will try to keep it simple here. We know that water and oil do not mix; the hydrophobic effect explains why. As we learned last week, water forms hydrogen bonds with other water molecules. When a non-polar substance (such as oil, can also be called a hydrophobe) is dropped into water, it cannot form hydrogen bonds with water. The water molecules surrounding the non-polar substance (called interfacial water molecules) must then orient to continue forming hydrogen bonds with themselves and so the water molecules form a "cage" around the non-polar substance. However, these water molecules forming the cage are more restricted than other water molecules and do not have as many available orientations (called a decrease in entropy or decrease in disorder), which is not energetically favorable. If two non-polar substances combine, the water cage around the larger combined non-polar substance will contain less water molecules than the two cages around the two individual non-polar molecules, allowing more water molecules to be free and the entropy (disorder) increases, which is energetically favorable. This is why the oil droplets clump together in the water. If you would like a more detailed explanation of the hydrophobic effect, read sections H6-H8 in Dr. Loren William's tutorial.
Q1: Using your own drawings (of the following molecule) and words, explain why it is energetically favorable for hydrocarbon chains to aggregate in water solution.

This carbon chain can also be depicted as a jagged line where each point represents one carbon and the carbon - hydrogen bonds and the hydrogen atoms are not shown. Because carbon always has four bonds the number of hydrogens are known just not drawn.

Q2: A detergent (such as SDS shown below which can be found in shampoo and toothpaste) contains a hydrophobic hydrocarbon tail and a hydrophilic head group. Explain how these detergent molecules would organize in a water solution and why this is energetically favorable.

pH and Buffers
pH (lower case p is used in chemistry to indicate the math function -log; pKa and pOH are other quantities that use this form) is the measure of how acidic (concentration of H+) or how basic (concentration of OH-) a solution is as
pH=-log[H3O+]. Read the rest of this page from Lumen Learning that introduces pH and buffers. If you need a more detailed review of acids and bases or pH, check out this website from the University of Texas.
Each acid and base has a dissociation constant (Ka) which defines how easily the molecule dissociates to form OH- or H+. Strong acids and bases have high dissociation constants which mean they almost completely dissociate (forming only the two individual ions). Weak acids and bases do not completely dissociate. For example, for the strong acid HCl:
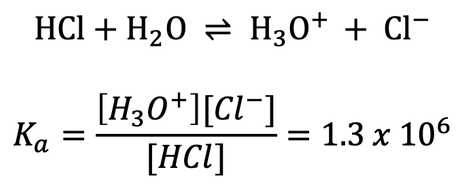
Buffers are solutions that resist changes in pH because they have large amounts of both the protonated (HA) and deprotonated (A-) forms of the acid (or base) and so are usually made of a weak acid and it's conjugate weak base.
When more acid is added, the solution will be neutralized by the deprotonated form.
When more base is added, the solution will be neutralized by the protonated form.
For example, your body’s pH is tightly regulated. Your blood pH stays between 7.35 and 7.45. If it gets too high or too low it can result in initial symptoms of weakness, dizziness, or nausea but can lead to coma and death if not treated.
Your blood's pH is buffered by carbonic acid (H2CO3, protonated, weak acid) and bicarbonate (HCO3-, deprotonated, weak base).
If acid is added it will combine with the weak base:
If base is added it will combine with the weak acid:



Q3: According to the chemical reaction below, what happens when the body has excess CO2? How would this affect the body's ability to buffer the pH of the blood? Can you think of a way the body could compensate for an increase in CO2?

Functional Groups
In this last section we will introduce functional groups. Functional groups are a group of atoms that have similar characteristics and reactivities and they are present in many molecules such as therapeutic drugs and proteins. We are interested in functional groups because they dictate what intermolecular interactions occur within and between molecules. When functional groups are drawn the common atoms are shown and the letter R is used to represent the rest of the molecule. If there are multiple R groups, then R', R'', etc. or R1, R2, etc. are commonly used. Here are many of the functional groups found in molecules:

Q4: Name three functional groups that are able to form hydrogen bonds.
Q5: What amino acid contains a thiol group? We don't expect you to memorize the amino acids or functional groups use all resources that you have encountered to answer questions we ask. To make this easier we show them below, but keep in mind we don't expect you to memorize anything and you can always refer back to previous lessons and materials.
Q6: What two functional groups do all amino acids contain?

"File:Amino Acids002.svg" by Pk0001 is licensed under CC BY-SA 4.0